- Printed Journal
- Indexed Journal
- Refereed Journal
- Peer Reviewed Journal

Journal of Pharmacognosy and Phytochemistry
Vol. 14, Issue 5 (2025)
Isolation and characterization of novel compounds from Curcuma amada Rhizomes: A phytochemical analysis
Lokesh Kumar Soni
Curcuma amada Roxb., known as "Mango ginger," is a perennial aromatic herb of the Zingiberaceae family from the Indo-Malayan region. This plant is significant in traditional medicinal practices, such as Ayurveda and Unani. The rhizomes possess antibacterial, anti-inflammatory, antioxidant, anticancer, and antidiabetic properties. This study focused on isolating and identifying novel compounds from C. amada rhizomes using chromatographic and spectroscopic methods. Methanol extraction of rhizomes was followed by purification using column and thin-layer chromatography (TLC). Two novel compounds were isolated, and their structures were determined using High-Resolution Mass Spectrometry (HR-MS), Fourier-Transform Nuclear Magnetic Resonance (FT-NMR), and Fourier-Transform Infrared Spectroscopy (FT-IR). The first compound, 5, 8-dihydroxy-7-methyl-4-oxo-1-hydronaphthalen-1-yl 4'-ethyl-2', 5'-dimethylhexanoate, was isolated as a dark brown sticky substance with a molecular ion peak [M+] at 360.16 (C21H28O5). The second compound, 8-dihydroxy-7-methyl-4-oxo-1-hydronaphthalen-1-yl 5'-hydroxy-2', 4', 5'-trimethylhexanoate, appeared as a dark brown sticky substance, with m/z 362.14 (C20H26O6). The 1H and 13C NMR spectra provided structural analysis data. This study first isolated these compounds from C. amada Rhizomes, warranting further research into their biological activities.
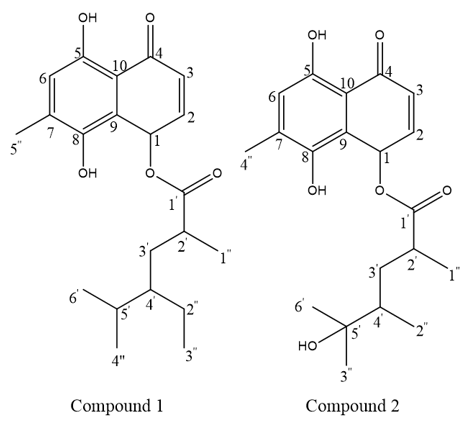
Fig. 1: The image illustrates the chemical structures of Compound 1 and Compound 2, which are derivatives of anthraquinone skeletons substituted with different functional groups. Both compounds share a common backbone consisting of a fused aromatic ring system with hydroxyl (-OH) and carbonyl (C=O) functionalities, characteristic of anthraquinone derivatives. Compound 1 contains hydroxyl groups at positions C-5 and C-8, along with a methyl substituent at C-6. It also possesses a branched aliphatic side chain attached via an ester linkage at C-1′, which contributes to structural diversity. Compound 2, on the other hand, displays a similar aromatic framework but differs in its side chain substitution, where an additional hydroxyl group is incorporated at C-3′, making it more hydrophilic compared to Compound 1. These structural differences may influence their biological activities, solubility, and pharmacological properties. The diagram highlights the structural diversity of naturally occurring anthraquinone derivatives and their potential therapeutic significance.
Pages: 102-107 | 585 Views 15 Downloads







